Second to electric motors, pumps are the most common industrial machine. They’re found in every conceivable industrial application processing all manner of fluids from waste water to paper pulp, pharmaceutical slurries to crude oil.
[pullquote]Process pumps in the pharmaceutical, food and beverage industries are subjected to stringent cleaning regimes, such as clean in place (CIP) and steam in place (SIP) to reduce and, hopefully, eliminate the possibility of bacteria and microorganism growth on surfaces in contact with the processed media. Such contamination is detrimental to the finished processed product and therefore considerable effort is undertaken by plant operators, engineers and managers to avoid it. But how successful are such cleaning regimens, specifically around the restricted and confined spaces of the pump’s all-important mechanical seal? Clearly, the answer to this question is dependent on the design of the mechanical seal cavity and, in particular, the components in the cavity that contact process fluid.
Hygienic seal design considerations
The main causes of pump process fluid contamination in hygienic applications are:
- Microorganisms entering the process fluid from the atmospheric side of the mechanical seal through the seal fluid film
- Process fluid passing through the seal fluid film to the non-process fluid side leading to microorganism growth in the residues. These microorganisms then move back across the seal fluid film into the process
- Process fluid sticking to the seal’s process wetted components or seal cavity when these areas aren’t cleaned correctly between processed batches
The European Hygienic Engineering and Design Group (EHGDG) is the main international body that provides best-practice guidelines for mechanical seal design considerations used in hygienic and aseptic applications. The EHEDG classifies hygienic equipment into three groups:
- Aseptic
- Hygienic Equipment Class I
- Hygienic Equipment Class II
Across the categories, the EHEDG stipulates that the sealing device should protect the process fluid from environmental contamination and be easily cleaned to prevent microorganism growth. It’s clear that equipment that is difficult to clean results in an inferior processed product, which might result in a rejected product batch, additional reprocessing costs, product recall and represent a danger for human consumption or use.
From the three equipment categories, Class I and Aseptic equipment requirements detail the use of an external seal to produce a buffer area (seal cavity), which can be flushed continually with appropriate fluid. This double seal requirement is intended to reduce the possibility of cross-contamination and problems arising from process fluid traveling across the seal fluid film of the inboard seal faces.
EHEDG also stipulates that unavoidable gaps must be sited at the seal’s non-process fluid side and the components in contact with the process fluid must be smooth and crevice-free. Again, this guideline is intended to reduce bacteria and microorganism growth potential from process fluid stick conditions, specifically between batches.
A million miles apart
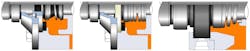
Reality and best practices rarely coincide. Unfortunately, many seals used in food, drink and pharmaceutical applications should be hygienically designed but aren’t; far from it. Single-coil component seal designs of the 1970s and 1980s are still dominant and in common use in the industry. Figure 1 shows a typical double face-to-face component mechanical used in a typical hygienic application.
Figure 1. Traditional double-component mechanical seal.
The process fluid (in orange) is in direct contact with multiple seal crevices and cavities in the single-coil spring and seal face drive mechanism in the processed media. These cavities are impractical if not impossible to clean thoroughly. This design ignores practically every international best practice seal design guideline and, unfortunately, this is not an isolated case.
Figure 2 illustrates three of the double-component seal designs used extensively in the food and drink industry. These designs have significant bacteria and microorganism growth potential around the process fluid wetted components. This fundamental problem is caused by the design of the component seal technology selected.
Figure 2 – A range of traditional double-component seal designs are used in hygienic applications.
An alternative is available. Plant engineers don’t need to take risks with these types of designs any longer. Upgraded geometries are now available and make such designs more hygienic. Putting these seals on a par with international best practice guidelines is neither difficult nor expensive.
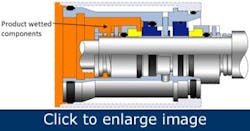
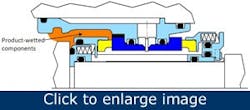
One mechanical seal design has won favor with many end users in the food, drink and pharmaceutical industries because of its practical solutions to these age-old hygiene issues. Figure 3 illustrates the same functional concept as Figure 1, yet the seal design conforms to the international best practice guidelines stipulated by the EHEDG.
Figure 3 – A double cartridge seal design for use in hygienic applications (AESSEAL plc).
This seal design reduces the possibility of process fluid contamination as the components the process fluid wets are hygienically profiled with smooth surfaces, sealed cavities and sterile rings in the elastomer grooves. It’s common sense that components with smooth surface finishes aid the cleaning and sterilization operations. Eliminating crevices and cavities makes it difficult for microorganisms and bacteria to adhere to surfaces. As one would expect, seal springs and drive mechanisms are positioned out of contact with the processed media.
Furthermore, best-practice engineers prefer cartridge seal designs as the one-piece design ensures the correct mechanical seal installation; something that’s essential to a hygienic seal. The EHEDG promotes such designs.
Failure to embrace modern seal technology, such as hygienically-profiled cartridge mechanical seals, introduces risk and uncertainty for any business processing sensitive fluids. Higher levels of risk increase the probability of problems arising for both the processing company and, ultimately, for human consumption and use.
Single-coil component seals might, on the surface, appear to be cheap, low-cost sealing solutions. However they have many failings. Best-practice companies recognize the hidden costs and risk associated with using such designs and increasingly look to innovative seals to deliver what international guidelines dictate.
The message is clear. Not all mechanical seal designs are the same. Continuous-improvement engineers need to look beyond the seal design supplied in the past for a specific application and look to what seal designs can be supplied today.
A. Roddis is engineering director at AESSEAL plc in Rotherham, Yorkshire, UK. The company’s U.S. branch is located in Rockford, Tennessee. Contact him at [email protected]; or www.aesseal.com; or (Tel.) +44 (0) 1709 514054.