In industries such as chemical processing, hydrocarbon refining, and power generation, leakage from extreme temperature process streams can result in loss of efficiency and production as well as adverse environmental impacts and compromised employee safety. One of the most commonly used sealing products in systems subject to high pressures and temperatures is a spiral-wound gasket. These gaskets typically consist of filler and winding materials selected on the basis of application requirements and end-user preference. Proper selection of these materials is critical to achieving the desired performance in all applications.
Material selection
Sealing at temperatures above 850oF (454oC) is particularly challenging because of the limited number of filler materials that can resist thermal degradation at extreme temperatures – these temperatures affect both the sealing material and metal components. For instance, the yield strength of fasteners decreases as the temperature is increased. In addition, certain chemicals can become more volatile and aggressive in high-temperature reaction processes.
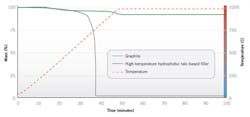
The two most common filler materials in spiral-wound gaskets are graphite (can withstand temperatures up to 850ºF) and polytetrafluoroethylene (PTFE; tolerance up to 500ºF). Other filler materials are used mainly for their thermal insulating properties, not for sealability, include mica, exfoliated mica and ceramics. While graphite and PTFE perform satisfactorily in terms of temperature and chemical resistance, they have limitations. Graphite is not compatible with heavily oxidizing media at any temperature; nor can it withstand continuous operating temperatures above 850oF. Beyond 850ºF, volume loss through oxidation becomes excessive and sealing effectiveness is compromised.
Many high-temperature systems, such as exhaust manifolds and flanged piping connections in exhaust systems, are oxidizing. Other services are oxidizing because of the operating temperature and media involved.
For example, molten salts have become popular as heat-transfer media for high-temperature processes and solar power generation. The temperature at which molten salts provide optimal heat-transfer performance is 1,049ºF (565ºC), creating a perfect storm in terms of oxidizing capability.
The only other filler that performs well in oxidizing media is PTFE, which offers exceptional chemical resistance in both oxidizing and nonoxidizing environments. However, it is limited to a maximum operating temperature of 500oF (260oC).
Binders and bonding agents
The addition of binders makes filler materials flexible enough to be manipulated in the manufacturing process and robust enough to withstand rough handling in the field. Graphite can be exfoliated and recompressed and PTFE can be sintered together, but nongraphite materials such as mica, vermiculite, ceramics and talc require a bonding agent to hold the particles and fibers together. Once the gasket is assembled and properly installed, the bonding agent has served its purpose. At this point, it will carbonize or burn off completely, depending on the service temperature. The loss of the bonding agent correlates to loss of mass, which can adversely impact sealability.
One method for measuring this impact is to check the sealability of a material under ambient conditions, expose it to elevated temperatures (i.e., 850ºF -1,000ºF) for an extended period of time, and repeat the initial sealability test. Loss of organic bonding agents would likely translate into a loss of sealing performance.
As noted, the metal components of spiral-wound gaskets likewise must be able to withstand elevated temperatures and be compatible with the service. Common winding metals include but are not limited to 304, 304L, and 316L. Depending on their size, spiral-wound gaskets can be manufactured horizontally or vertically. Spot welds secure the metal windings for structural integrity and prevent them from unraveling. In addition, inner and outer rings can be installed with the windings to guard against damage during handling, installation and use.
Manufacturability
In manufacturing spiral-wound gaskets, continuous lengths of filler material have to be produced in a way that allows the material to be wound in plies between alternating layers of preformed metallic wire.
Converting the raw filler material into usable solid sheets typically involves a sheet or roll forming process, which precludes many high-temperature materials from being used in the spiral winding process.
The ability to exfoliate or expand and densify graphite enables production of rolled sheets of high-purity (>99%); these can be slit to varying widths depending on the required thickness of the filler material. However, the quality of the raw graphite can significantly affect how well it processes and performs in service. Lower-grade graphite can contain higher levels of impurities that will oxidize at lower temperatures, resulting in mass loss and compromised sealability.
The most widely specified standard for metallic gaskets such as spiral wounds is ASME B16.20-2012, Metallic Gaskets for Pipe Flanges. This is an industry-accepted standard that specifies dimensions, tolerances, marking requirements and method of construction for spiral-wound gaskets, as well as grooved metal gaskets (kamm profiles), ring-type joint gaskets and double-jacketed (DJ) gaskets. Due diligence must be performed to confirm that gaskets with different fillers comply with such manufacturing standards.
Logistical integrity
As noted, finished gaskets need to maintain their integrity during shipping, handling, storage, and installation. This “logistical integrity” is critical. A commonly overlooked factor that affects gasket performance is moisture absorption. Some filler materials are more hydrophilic or susceptible to absorbing moisture, which can pose a major problem if they are exposed to chemicals that react with water or elevated temperatures that could cause the moisture to flash off.
The best way to prevent damage from moisture absorption is to use gaskets with hydrophobic fillers. Figures 1-4 show gaskets with two different filler materials: Gasket A has a vermiculite filler, and Gasket B has a talc-based filler. The figures depict the gaskets after exposure to water for 40 minutes (Figure 1), 4.5 hours (Figure 2) and 7 hours (figures 3 and 4).
The integrity of the windings is critical to effective sealing. After the immersion test, Gasket A, filled with vermiculite material, was completely compromised as the filler absorbed an excessive amount of moisture, swelled and flaked off. Gasket B, filled with a recently developed hydrophobic talc-based material, did not absorb moisture and maintained its integrity and sealability. The high-temperature filler material used in Gasket B is designed to provide sealing effectiveness at temperatures up to 1,832oF (1,000oC), including extreme thermal cycling conditions.
Because of its low organic fiber content and nonoxidizing formulation, the new material exhibits only minimal loss of mass at these extreme temperatures, ensuring proper load retention is maintained while the gasket is in service. Its oxidation resistance and thermal stability at extreme temperatures make this material suitable for critical service conditions over 850ºF and with oxidizing media.
The graph below (Figure 5) shows how temperature affects the integrity of traditional graphite as compared with the new high-temperature material. The test was performed using a thermal gravimetric analyzer, which measures weight loss over time and temperature.
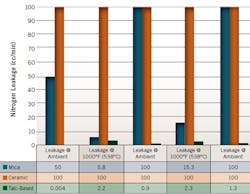
It is critical to consider not only installed performance but also qualitative metrics such as logistical integrity when selecting a gasket. A gasket's purchase cost is insignificant compared with the consequences a plant will face if a gasket fails because the wrong type was selected for the job. The graph below (Figure 6) shows how the new high-temperature material performs compared with mica and ceramic materials. The new material seals well not only at extreme temperatures, but also under conditions of thermal cycling.
Wayne Evans is a product engineer and IP lead at Garlock Sealing Technologies, where he focuses on low-friction compression packing, product development for solid oxide fuel cells and various high-temperature sealing solutions for alternative energy applications. He has four patents, two of which are pending. Contact him at [email protected]. Matt Tones is senior applications engineer for gasketing at Garlock Sealing Technologies. He also has served as director of product management for North America; manager of applications engineering, training and customer support; product manager for restructured PTFE gaskets; and OEM liaison. Contact him at [email protected].
A few dominant characteristics – the most important being the stress at which they seal – determine the effectiveness of spiral-wound gaskets. Some gaskets create effective seals at lower stresses; this is an important consideration for extremely hot applications where the flange bolting will not permit higher-compressive stresses. Sealing manufacturers are an excellent resource for information regarding loading conditions for an effective seal.
When considering any sealing product, it is important to take into account all of the components that go into it and validate how the final selection will be affected by the handling, storage and environmental conditions. Sealing manufacturers are being challenged to develop innovative solutions to meet the increasingly demanding conditions of industrial applications where operating temperatures continue to be raised to improve process efficiency and yield. For gaskets, that means going beyond the performance of traditional materials.