How hydroxyl radicals can improve plant safety and performance
Have you ever heard of atmospheric hydroxyl radicals? They are the most remarkable molecule you’ve never heard of. They are the reason the Earth’s atmosphere does not accumulate most chemicals (other than a handful of greenhouse gases). Hydroxyl radicals are non-toxic to humans, animals, and plants, yet they kill bacteria, virus, and mold. Because they are naturally occurring there are no regulatory issues with deploying hydroxyl radicals.
Note: There are actually four types of hydroxyl radicals: atmospheric, in-vivo, chemical, and interstellar. This article only discusses atmospheric hydroxyls. In-vivo refers to hydroxyls that are formed inside the human body. They are formed through a Fenton reaction as a body’s response to severe diseases. Interstellar are simply hydroxyls in outer space.
Our current atmosphere has existed for approximately 400 million years. It has fluctuated some over time but currently consists of about 78% nitrogen and 21% oxygen, with argon and some other gases making up the other 1%. Carbon dioxide, for instance, is only 0.0385%. The atmosphere also contains water vapor, which we know as humidity.
It was in the 1970s that studies were conducted that looked into why some chemicals accumulated in Earth’s atmosphere and others didn’t. After a great deal of research it was determined that atmospheric hydroxyl radicals were the dominant reason for the decomposition or neutralization of chemicals in the air.
Why should you care about atmospheric hydroxyl radicals? They destroy volatile organic chemicals (VOCs). They destroy viruses. They destroy bacteria. They destroy mold. Hydroxyl radicals destroy these chemicals and micro-organisms throughout indoor air and on surfaces. They do not penetrate liquids or solids, which makes them ideal for food & beverage, drug manufacturing, research facilities, hospitality facilities, and many other environments.
Because hydroxyl radicals destroy VOCs and micro-organisms they can reduce downtime costs for sanitation, improve indoor air quality for the health and safety of employees, and improve product quality so more products can be sold at lower costs. In healthcare settings, hydroxyl radicals can reduce the incidence of infections, which lowers costs and improves patient health and throughput.
Since the 1970s there has been research into producing hydroxyl radicals within indoor or enclosed spaces. NASA developed a hydroxyl generator unit to be used on spacecraft using ultraviolet (UV) energy and titanium dioxide (TiO2). Others have developed equipment that requires UV energy and supplemental chemicals to be added.
These devices produced very weak streams of hydroxyl radicals that were ineffective in treating large indoor volumes. Over the past 10 years, hydroxyl generators that use only a photolytic process have been developed. They can produce hydroxyl radicals indoors or in any enclosed spaces. This newer class of equipment requires ambient humidity (ideally above 40%) and 115v or 220v power. These devices produce super-charged hydroxyl radicals that can be projected into spaces to sanitize the air and surfaces. In many cases they can be retrofitted into existing ventilation systems, or they can be standalone units within the space.
What exactly are atmospheric hydroxyl radicals?
The portion of the Earth’s atmosphere that faces the Sun receives the Sun’s UV energy. When the UV energy impinges on water molecules in the atmosphere there are a couple of chemical reactions that occur. Among those reactions is the cleaving of one of the two hydrogen atoms from the water molecules. The result is an elemental hydrogen atom and a negatively charged oxygen and hydrogen molecule. The negatively charged OH molecule (∙OH) is the remarkable molecule, or the hydroxyl radical (or simply hydroxyl).
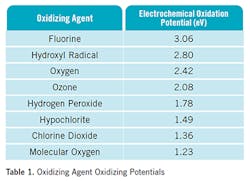
Atmospheric hydroxyl radicals are excellent oxidizers, second in oxidizing strength behind fluorine. Table 1 shows various oxidizers and their relative strength. Oxidation means the loss of or transfer of electrons. In the air, oxidation generally involves the reaction of chemical species with an oxygen containing molecule. There are very few molecules in the atmosphere that withstand or only slowly react with hydroxyls. Chlorofluorocarbons (CFCs), nitrous oxide (N2O), carbon dioxide (CO2) and methane (CH4) are examples. These are known as greenhouse gases. CFCs had been used extensively, but because they don’t oxidize, and because they attack the ozone layer, they have been replaced with substitutes that contain hydrogen atoms. This makes them susceptible to oxidation by hydroxyls.
In 1987 NASA conducted a study to measure the concentration of hydroxyl radicals in the atmosphere. The measurements revealed that there were between 500,000 and 2,600,000 hydroxyl radicals per cubic centimeter on a sunny day. The concentration varies with altitude and latitude, and the amount of water vapor and sunlight. At sea level on a sunny day the concentration is less than 1 part per trillion. However, this low concentration is ubiquitous – it is available over the Earth’s surface that is in daylight.
In 1995 the Intergovernmental Panel Conference on Climate Change acknowledged that atmospheric hydroxyl radicals were responsible for cleansing methane from the atmosphere. Methane is recognized as a contributing factor in the destruction of the ozone layer. As stated above, the oxidation of methane is very slow, but hydroxyls do destroy methane over time.
Why should plant and facility managers care?
The atmospheric hydroxyl radical molecule’s impressive oxidizing strength can be applied to many plant or facility challenges. There are many processes such as composites manufacturing (VOCs) or food & beverage industries (requiring high levels of sanitizing chemicals) that can produce toxic vapors or fumes that are harmful to humans. Hydroxyls can knock these harmful chemicals out.
In industrial hygiene the mantra is “destroy, dilute, then PPE.” This means it is best to destroy the toxic or undesirable substance. Next best is to dilute the concentration of the substance to an acceptable level. The least recommended approach is to require PPE.
The best answer for many applications is to destroy the substance, which can be done with hydroxyl radicals. When you dilute the substance, perhaps by having greater air exchanges, there are increased expenses for ventilation fans, heating, or cooling costs increase, more filter replacements, etc. The offensive substance may also be transferred to the environment, just in diluted fashion. PPE has the potential of not being available and not being properly serviced, not properly worn or deployed.
In one example at a food & beverage facility, background levels of oxidants were measured prior to installation of hydroxyl generators. It was found that the sanitizing chemicals were being used in such a way that the level of oxidants exceeded the threshold limit value (TLV) for worker exposure. By installing hydroxyl generators and maintaining the ambient level of hydroxyls below 8 parts per million, the other sanitizing chemicals were drastically reduced. Total oxidant levels ended up well below the TLV.
Hydroxyls attack micro-organisms through a different mechanism. The way hydroxyls attack micro-organisms is through a process called cell lysing, which occurs when a hydroxyl electric charge (in millivolts) comes in contact with the micro-organism’s cell membrane. The electric charge ruptures the membrane killing the micro-organism. It turns out that cell lysing is extremely effective in killing virus, bacteria, and mold.
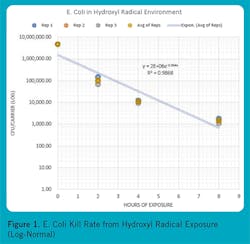
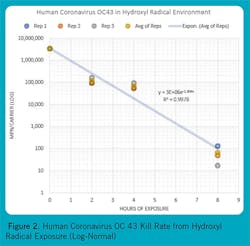
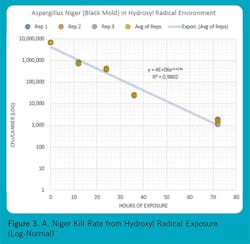
Figures 1, 2, and 3 show some reference micro-organisms that were tested by an independent biological sciences laboratory. The reference micro-organisms include Escherichia Coli (ATCC1 11229, E. coli), Human Coronavirus OC 43 (ATCC VR 1558), and Aspergillus niger (ATCC 16404, black mold). Note the high kill rates and short exposure times for E. coli and Human Coronavirus OC 43. Black mold is the toughest mold to kill, but it was subdued over several days.
In a plant or facilities environment being able to address toxic or odorous chemicals and killing micro-organisms with hydroxyl radicals has several advantages:
- Hydroxyls are non-toxic to people, animals, or plants. In fact humans, animals, and plants have all evolved in a hydroxyl rich environment.
- Hydroxyls do not damage plastics, elastomers, cloth, or metals.
- Photolysis-based hydroxyls are a green technology. It requires no chemicals to be used in this process.
- Hydroxyl production can be monitored and adjusted to ensure equipment is run efficiently and no energy is wasted.
- Toxic chemicals and strong odors from existing production processes can be destroyed.
- Continuous hydroxyl sanitation reduces the volume of other chemical cleaners and can improve the health and safety of the cleaning/sanitation crew. This can help put employees at ease about returning to the workplace and helps in attracting and retaining employees.
Some of the industry-specific benefits include:
- For food & beverage, bio-research, medical equipment, pharmaceutical and other processes with high sanitation standards, production availability can be increased by reducing the time needed for sanitation to increase production availability.
- Product quality can be improved by reducing contamination from virus, bacteria, and mold resulting in more saleable product, with higher revenues and profit. Endotoxins that off-gas from dead micro-organisms are just VOCs that are decomposed with hydroxyl radicals.
- For healthcare spaces such as hospitals, clinics, and senior assisted living facilities, continuous sanitation can reduce the spread of virus and bacteria such as Clostridium difficile (C. difficile) and methicillin-resistant staphylococcus aureus (MRSA) and coronavirus.
- Public spaces such as hotels, universities, public transportation, restaurants, and retail stores can use hydroxyls to ease apprehensions of employees and visitors. This can lead to increased use and additional revenues.
Naturally occurring hydroxyls exist in outdoor environments during daylight hours. Therefore, naturally occurring hydroxyls are not available inside buildings or during evening hours. However, by mimicking the natural processes, hydroxyls can be created in an indoor environment. To create them, we simply need ambient air (ideally with 40% relative humidity or more), and then apply specific UV wavelengths in a specific sequence.
In fact, using the photolysis process, additional energy can be imparted onto the hydroxyl radicals. This results in a higher capacitance which enables chain reactions of hydroxyl radicals. This is called the cascading effect. The cascading effect allows a generated hydroxyl to flow into a space and impart enough energy to create a chain of new hydroxyls. In this way an entire space can be flooded with hydroxyl radicals and can be safely and efficiently treated.
Because of the cascading effect, hydroxyl generators can treat large volumes of space. Often, hydroxyl generating equipment can be retrofitted or designed into heating, ventilation, and air conditioning (HVAC) systems. Standalone units can be placed in the space with its own circulation fan. Hydroxyl generating equipment can be scaled to treat any volume of space.
Here are a few examples of the range of situations that can or have benefited from hydroxyl radical treatment:
- brewery filling lines
- chicken and turkey egg laying, egg hatching and grow farms
- grain and grain by-product bacteria and mold treatment
- indoor agriculture bacteria, mold, and odor
- manufacturing central machining coolant odor
- hog barn hydrogen sulfide reduction
- wastewater treatment facilities and lift station hydrogen sulfide reduction
- pre-sanitization of tools, equipment and parts that need to be brought into sterile work environments (food & beverage, research facilities, vivariums, drug manufacturing, ICUs)
- public transportation.
An example business case: Brewery filling line
The brewery had a couple of sold out products. It could sell as much as it could produce. One particular filling line was producing approximately 2,000 cans of beer per minute. During the filling process there is some spillage that occurs within the filling line enclosure. Because ambient air contains mold spores, and because the temperature and humidity are ideal for growing mold, the line had to shut down every four days for an eight hour shift, for cleaning, sanitation, and sterilization.
Brewery profit margins are about 8%. If you assume the wholesale price for each can of beer is $1.00 that means each can of beer is worth eight cents in profit for the brewery. At 2,000 cans per minute and 60 minutes per hour the downtime cost of lost production is $9,600 per hour. But the shutdown period was eight hours or $76,800. This does not include the shutdown and startup losses, which would drive this amount upwards.
After extensive health, safety, and quality testing, hydroxyl radicals were applied to the filling line. The installation required the hydroxyl radical generator equipment, some ductwork, electrical connections, and a Wi-Fi connection. Once the system was commissioned, it was observed and confirmed through micro-organism testing that the bacteria and mold were killed. At the four-day periodic shutdown the time needed was cut in half. After the hydroxyls began doing their work the line was being shut down for just four hours instead of eight hours. The production losses were also cut in half to about $38,400 per shutdown.
Subsequently, the filling line shutdown periods underwent age exploration. They extended the intervals from every four days to five days, then to six days. The system had a payback period of less than three months.
As mentioned above, there were other benefits. The high background level of oxidants from the other sanitizing agents were reduced, improving the safety and health of employees. Reducing the other oxidizers further reduced the cost of sanitizing chemicals and reduced rouging of stainless steel equipment. In the process of assessing the filling line application, the waste recycling room was identified as a contributor to mold distribution. Forklifts were moving back and forth through the filling line area and recycling room. Hydroxyls were recommended to treat the recycling room to reduce mold spores being transferred into production areas.
Why haven’t you heard about hydroxyls before now?
There are several reasons why you’ve not heard about the remarkable molecule. First, the development of photocatalytic hydroxyl generators (a method that requires additional chemicals) has only been done within the past 23 years. Photocatalytic hydroxyl generators are unable to generate the cascading effect. Because of that, the application to large spaces was unavailable.
Second, manufacturers of early photocatalytic hydroxyl generators lacked sufficient experience in working with manufacturing, industrial, and utility customers. They struggled to develop the messaging and branding to make the technology well-known.
Third, the projects that early photolytic hydroxyl generator companies engaged in were with innovators and early adopters. These customers required confidentiality agreements that slowed the market adoption of the technology.
The reason for this article is to encourage plant managers, facilities managers, operations managers, maintenance and reliability managers, sanitarians, risk managers, infection control professionals, and others to become familiar with photolytic generation methods for atmospheric hydroxyl radicals. Learn more about how they can be applied to solve problems, increase revenues, reduce costs, and improve health and safety for employees and customers.
Radical Clean Solutions, Ltd. was formed to make hydroxyl radicals better known, and applied, throughout the world. We are working diligently to form partnerships with large organizations that will help disseminate information, support applications, and make hydroxyl radicals a widely accepted solution. This includes manufacturing, industrial, pharmaceutical, food and beverage, utilities, facilities, and the service companies that support them.
One such partnership is with AgriFORCE Growing Systems. Ltd. (AgriFORCE). AgriFORCE develops and commercializes industry-disruptive, foods, specialty ingredients, and technologies. They are experts at controlled environment agriculture (CEA) with extensive intellectual property. AgriFORCE is working with Radical Clean Solutions to start commercializing a set of solutions, which address some of the most important problem areas in the CEA and food manufacturing sectors. It is estimated that mycotoxin (mold) affects nearly 25% of the world’s crops.
In addition to mold, airborne pathogens, such as COVID-19, contribute to significant loss of personnel due to sick leave, which is evident by the massive supply chain issues that have affected the industry during the current pandemic. Moreover, by destroying bacteria such as E. coli and Salmonella, food producers and manufacturers can potentially avoid costly recalls, which have both a financial and lasting brand impact.
Finding out more
My contact information is listed at the end of this article. You’re encouraged to contact me with your questions. You can also do your own research. Every year there are more and more universities, research centers, and companies that are studying and finding more information on atmospheric hydroxyl radicals. I encourage you to do your due diligence. Be a skeptic. But when you convince yourself of the opportunity, seize the day. Be an innovator or early adaptor. Health, safety, production availability, and business performance will improve.
[1} ATCC (American Type Culture Collection) is a private, nonprofit, global biological resource center and standards organization that provides scientists with the biomaterials and resources they need to conduct critical life science research.
This story originally appeared in the August 2022 issue of Plant Services. Subscribe to Plant Services here.