Many plants use small to medium volumes of hydrogen for semiconductor manufacturing, materials processing, generator cooling, chemical production, and other applications. Hydrogen is highly valued for its extremely low density, high thermal conductivity and powerful chemical reducing properties. Until recently, small volume users had few supply options other than storing compressed or liquefied hydrogen. Technological advances now allow users to generate high purity hydrogen on-site economically as needed.
|
View more content on PlantServics.com |
In addition to its industrial uses, hydrogen is being considered as a fuel for vehicles, home electricity production and portable power applications,ranging from cell phones to portable generators. As a fuel, hydrogen is unique in that it can be processed in a fuel cell to make electricity without combustion, yielding only water vapor and a small amount of waste heat as byproducts. Fuel cells operate at about twice the efficiency of combustion devices (such as an engine generator) because they waste less energy as heat. Even when fueling a burner or internal combustion engine, hydrogen combustion yields no pollution other than part-per-billion levels of nitrogen oxides.
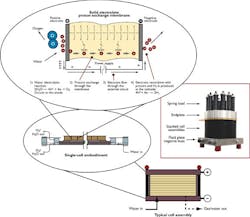
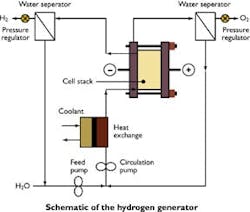
Hydrogen is the most abundant element in the universe, and the most abundant reservoir of terrestrial hydrogen is the oceans. The earliest hydrogen supply method,splitting water molecules electrolytically,makes highly pure hydrogen, at large or small scale, by using electricity from any source from nuclear to coal to solar photovoltaic to wind. Electrolysis directly from sunlight can position hydrogen as a possible motor fuel,a possibility for truly renewable energy. While it is highly valued for its simplicity, electrolysis has been a niche production technique.
Most of the hydrogen used by industry is not made from water, but from reacting the methane in natural gas with water vapor to produce hydrogen and carbon dioxide, which are then separated. The driving force for this reaction is heat generated by burning some of the natural gas. This technique has been favored over electrolysis for large industrial applications because it uses low-cost natural gas as the energy source instead of valuable and often expensive electricity.
Generally, only large chemical plants split natural gas to make hydrogen. Trucks or pipelines then transport the gas to users. The total cost of delivered hydrogen includes the costs of production, compression or liquefaction, and delivery. Historically, trucking hydrogen has been less expensive than making hydrogen on-site with electrolysis. The technology to produce small amounts of highly purified hydrogen cost-effectively onsite using a natural gas feedstock has not proved feasible because the required purification steps do not scale down in a cost-effective manner.
New thinking
The use of natural gas as the primary hydrogen feedstock is being challenged by new circumstances. Some of these include:
- The rapidly changing deregulated electricity market, which is making electrical power more cost-effective for many users.
- Rapidly increasing distribution costs for trucked hydrogen because of fewer available drivers, crowded roads, limited customer delivery hours, and increased insurance costs.
- Hydrogen is a difficult product to transport cost-effectively. A 40,000-lb. trailer of the compressed gas contains only about 600 pounds of hydrogen. Even a trailer with 13,000 gallons of liquefied hydrogen transports less than 8,000 pounds of usable cargo.
- Heightened concerns about flammable gas storage and delivery of hazardous materials make it harder to store hydrogen inventory.
- Calls for reduced carbon dioxide emissions, which are significant with natural gas but potentially zero with electrolysis.
- Geographically isolated locations.
- When very high purity gas is required.
- Inexpensive electricity is available.
- Very small quantities of gas are required.
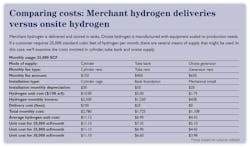
The cost of electrolysis is easily understood. It includes the cost of equipment purchase, installation (amortization plus interest), equipment maintenance, electricity and water. Because hydrogen is made on-site, there is no delivery cost. Even so, until recently, the total still exceeded the cost of delivered hydrogen.
Advances in standardization, simplification, and packaged design have made electrolysis a more cost-effective technique. Equipment is also more compact, easier to operate and virtually maintenance-free,which reduces the complexities of installation, operation and maintenance. These changes have made onsite electrolytic hydrogen generation an attractive option for the end-user.
[pullquote]Modern electrolysis equipment can replace cylinder hydrogen with total costs less than the cylinders replaced. For example, a hydrogen generator that provides 40 standard cubic feet per hour (SCFH) of ultra-high purity (99.999+ percent) hydrogen at 200 psi is about as large as a dishwasher. It produces hydrogen for a total cost of less than $5 per 100 standard cubic feet, which is often far less than the cost of cylinder gas. Larger systems produce hundreds of standard cubic feet per hour for less than $3 per 100 standard cubic feet.
The total cost for a hydrogen generator includes the capital cost, installation cost, operating cost (chiefly electricity), periodic maintenance, plus the cost of a backup gas supply in case of electrical interruption. Onsite production eliminates delivery and inventory uncertainties, provides consistent high purity, eliminates make and break connections and simplifies the cost structure with a single monthly invoice.
Packaged electrolysis systems are sized appropriately for a number of applications, including electric generator cooling, materials processing, semiconductor fabrication, electrochemical research and small scale chemical processing. For customers using small-scale hydrogen applications, onsite hydrogen generation brings new convenience, safety and economy.
These forces and others create an opportunity for electrolytic hydrogen to be considered again as a cost-effective supply. Current technology is based on the premise of using small, distributed onsite plants to supply hydrogen directly to a process and eliminate the need for hydrogen delivery and storage.
Electrolysis requires only an electrolyzer, purified water and a source of electricity. Each pair of water molecules splits into one molecule of oxygen and two molecules of hydrogen. Electrolysis has been used for nearly a century in a number of instances such as:
How to determine your hydrogen consumption rate
When using cylinders or tubes of compressed hydrogen, or liquefied hydrogen stored in vacuum-jacketed liquid tanks, the supply system is capable of meeting virtually any rate of use. Generally, all that matters when sizing a system for stored hydrogen is the monthly average use rate. If the monthly use rate changes, the supplier simply delivers more or less often.
Onsite hydrogen production systems are sized to meet a certain peak rate and a total monthly production. It is important to understand both factors when sizing an onsite system.
Many applications, such as industrial electric generator cooling and metal heat treating, use hydrogen at a fairly steady rate for extended periods of time. If your plant has a process like this, simply divide the total monthly hydrogen use by 730, the number of hours in a month, to determine the average hourly use rate. Other applications exhibit a predictable peak in use. These applications, such as semiconductor manufacturing and oil processing, may require a much higher instantaneous flow rate, but offer the opportunity to idle between supply periods. In a situation like this, an onsite hydrogen generator may be teamed with a gas buffer storage system to meet demand during high use periods.
Whether the use rate is steady or has intermittent peaks, the place to start is with the monthly hydrogen invoice, which shows delivery dates and volumes received. Divide the volume by the delivery time interval to determine the average use rate. Next, examine each user to determine the instantaneous maximum required flow rate. Finally, determine how long peak rates might last to determine if they might be met through storage.
Keep in mind that the most economical gas supply solution, and the best one for your needs, may be a hybrid system that combines the best attributes of both onsite production and merchant delivery. You can meet the baseload (continuous, predictable) hydrogen requirement through onsite production and meet the short term, peak and unpredictable hydrogen requirements with delivered hydrogen. That way, most of the use is supplied by low-cost manufactured hydrogen without paying for excess production capacity.
Soft versus hard costs in hydrogen supply
The two major cost categories to consider when determining the most advantageous mode of hydrogen supply are the hard costs and soft costs. Hard costs are directly and obviously related to hydrogen supply. Examples include hydrogen system installation and monthly hydrogen delivery invoices. Soft costs are more general and include manpower, floor space, security, and insurance. These costs are real, but are much harder to attribute to any particular production process. It's harder to claim a savings involving soft costs.
Hard costs are the out-of-pocket expenses for hydrogen supply. They include:
- The unit cost of hydrogen deliveries or production (a variable cost).
- The cost of the rental or depreciation charges for rented or owned hydrogen systems.
- The depreciation cost for installed hydrogen distribution assets,foundations, fencing, piping, etc.
- The costs of fees paid for delivery, such as hazardous materials fees.
Add up the monthly or annual hydrogen cost reports to get a total that represents your hard costs. Be aware that your organization may report these costs in a way that makes them difficult to find. Storage rental, for example, may be on a report with other rental items, but separate from hydrogen related costs.
Soft costs are even harder to track and, for many organizations, it may not be worth the trouble to try to itemize these costs. It may be sufficient to simply say that time spent on changing cylinders, reconciling invoices, receiving hydrogen deliveries and other actions related to merchant deliveries will be saved. Often, an economic decision that relies on soft cost savings is too subjective to be approved by management.
The best approach to making the right decision, and getting that decision approved by management, may be to use only hard cost for justification, and save the soft costs to support an otherwise hard cost justification.
David Wolff, vice president, Proton Energy Systems, can be reached at [email protected].