Protective coatings are integral to maintaining any piece of equipment. Without effective coatings, water, chemicals or other environmental materials can damage parts and force costly, time-consuming repairs. Although everyone knows that quality protective coatings can keep equipment operating more efficiently and longer, not everyone knows exactly what constitutes a quality protective coating.
Many ingredients in a coating contribute to effective protection. Solvents and resins, in particular, can have a significant effect on a coating's ability to prevent corrosion, abrasion and other environmental damage.
As with any industrial coating, a protective coating must meet regulatory guidelines. The concentration of volatile organic compounds (VOCs) is regulated, and thus, it may be necessary to reduce or even eliminate VOCs in a formulation. The levels of environmentally hazardous and toxic materials must also be regulated. Formulations must meet these ever-changing regulations without compromising performance.
A quick look at several coating components shows how each may affect the coating's performance and the level of protection it provides.
Figure 1:
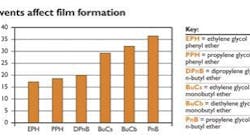
Of these solvents, EPH is most effective at reducing minimum film formation temperature (MFFT).Solvents aid film formation
Over the years, plant engineers have used many coating systems -- from low-solids, solvent-based coatings to two-component, waterborne, cross-linkable coatings. As equipment has changed, the coatings used to protect it have also changed, providing better protection and resistance to corrosion.
Solvents play a major role in the coating's protective abilities. Solvents solubilize the coating's film-forming ingredients. Solvents aid in film flow and leveling and keep the ingredients soluble during drying. The more effective the solvent, the more effective the coating is at keeping out materials that could corrode or damage substrate metals.
An emerging trend to meet this need is an increase in the use of high-solids, solvent-based coatings formulated with hydrophobic solvents. New, stronger, corrosion-resistant resin systems that are used with these coatings often require a polar solvent or one with other functionality to dissolve them easily. Glycol ether esters can accomplish this by offering polarity with less hydrogen bonding.
Waterborne latex coatings
Waterborne latex coatings are used to protect pipes and equipment housings. These coatings are easy to use and have favorable regulatory profiles. They're non-flammable, with low odor properties. To be formulated for industrial maintenance finishes, waterborne latex coatings use specialty resins, which increase hardness and improve resistance to corrosion and abrasion.
To facilitate the formation of a continuous film with these specialty resins, the coating must have an efficient coalescing solvent. Otherwise, the resin won't form a continuous film, allowing water to penetrate the coating.
The solvents used in a waterborne latex coatings must be water-compatible and have an affinity for the latex resin. Molecules such as dipropylene glycol n-butyl ether, propylene glycol phenyl ether and ethylene glycol phenyl ether are extremely efficient coalescents. Solvents that reduce film formation temperature -- a measure of coalescing efficiency -- can help improve the coating's protective capabilities. Figure 1 shows how different solvents reduce the minimum film formation temperature (MFFT) of an industrial acrylic resin.
Waterborne polyurethane
When chemical resistance is necessary, a cross-linked film often affords the most effective protection. Waterborne polyurethane topcoats, for example, offer excellent resistance to chemicals. The solvents in these formulations must be inert, water-soluble, and still be able to dissolve organic molecules. The solvent must dissolve the reactive isocyanate polymers and yet be miscible with water-based hydroxy functional components. Glycol diether solvents offer water solubility, excellent solvency, and they won't react with isocyanate functional groups. Glycol diethers also provide effective film formation once the film is cast, which allows the two components to react and form a highly cross-linked protective coating.
Resins -- the barrier
The resin in your protective coating dictates the performance attributes of your equipment. A thermoplastic resin is thicker and more flexible than a thermoset resin, but doesn't adhere well to metal, and can peel or blister allowing corrosive materials to get underneath the coatings. Peeling and blistering can be overcome with an adhesion-promoting metal pretreatment, but at an additional cost.
Thermoset resins not only adhere to metal, but provide effective protection in highly corrosive environments -- one of the main reasons they're the resin of choice in chemical or pulp and paper processing plants that routinely work with corrosives. Thermoset resins are used on storage and reaction vessels, process piping, fume hoods and ducts -- any surface that might come in contact with acids, bases, oxidizing agents or even hot gases.
In an application that requires excellent mechanical and thermal performance, thermoset resins, such as epoxies, polyesters and vinyl esters, are also good bets. They protect equipment from high pressure, vacuum and wind loading. If extreme temperature performance is required, brominated thermoset resins offer some of the best protection available.
VOC regulations
Solvent-based coating systems offer unsurpassed protection for plant equipment. However, changing regulations influence the types of solvent that formulators use.
Protective coatings are trending toward a reduction in solvent and using less toxic solvents in response to VOC and hazardous air pollutant (HAP) regulations. Many formulators are replacing weak solvents with more efficient solvents by matching solubility parameters; this reduces both the amount of solvent used and VOC emissions. Coatings are increasingly being formulated with non-HAP oxygenated solvents to meet new regulations without sacrificing performance.
As coating technology evolves, the role and choice of ingredients in industrial maintenance finishes must also change. Using multifunctional solvents with unique characteristics improves film formation, meets regulations and implements new technology. Oxygenated solvents from the glycol ether and ester families are strong bets for protective coatings, as they meet the performance needs of almost any coating, from waterborne latex to cross-linked systems. Meanwhile, the proper thermoset resin can provide added corrosion resistance, thermal and mechanical performance. Ask your coatings supplier and you'll be more likely to ensure solid protection for your plant equipment.
Dirk Zinkweg is a business development leader in Glycol Ethers Technical Service and Development for The Dow Chemical Co., Midland, Mich. More information is available at (800) 447-4369.
Figures: The Dow Chemical Co.